71 - Thoracic Lymphnodes Classification
*Thoracic lymph nodes are classified into 14 stations:
*Station number 1 : Low cervical, Supraclavicular and Sternal notch nodes.(R and L).
*Station number 2 : Upper paratracheal nodes (R and L).
*Station number 3 : Prevascular(3a) and Retrotracheal nodes (3p).
*Station number 4 : Lower paratracheal nodes (R and L).
*Station number 5 : Subaortic (aortopulmonary window).
*Station number 6 :
PHYSICAL CHARACTERISTICS OF BLOOD
PHYSICAL CHARACTERISTICS OF BLOOD
- Is denser and more viscous than water
- Feels slightly sticky
- Temperature - 38 °C - 100.4 °F
- slightly alkaline pH ranging from 7.35 - 7.45
COLOR
- Color varies with its oxygen content
- High oxygen content - bright red
- Low oxygen content - dark red
Constitutes
- 20 % of extra cellular fluid
- 8 % of total body mass
VOLUME
Adult male - 5-6 liters
Adult female - 4-5 liters
IMPORTANT HORMONES ENSURING BLOOD VOLUME AND OSMOTIC PRESSURE RELATIVELY CONSTANT
1. Aldosterone
2. ADH
3. Atrial natriuretic peptide
- Is denser and more viscous than water
- Feels slightly sticky
- Temperature - 38 °C - 100.4 °F
- slightly alkaline pH ranging from 7.35 - 7.45
COLOR
- Color varies with its oxygen content
- High oxygen content - bright red
- Low oxygen content - dark red
Constitutes
- 20 % of extra cellular fluid
- 8 % of total body mass
VOLUME
Adult male - 5-6 liters
Adult female - 4-5 liters
IMPORTANT HORMONES ENSURING BLOOD VOLUME AND OSMOTIC PRESSURE RELATIVELY CONSTANT
1. Aldosterone
2. ADH
3. Atrial natriuretic peptide
BLOOD PLASMA
COMPONENTS OF BLOOD
1. BLOOD PLASMA
2. FORMED ELEMENTS
BLOOD PLASMA
- 55 %
- WATERY LIQUID EXTRA-CELLULAR MATRIX
- CONTAINS DISSOLVED SUBSTANCES
FORMED ELEMENTS
- 45%
- CELLS AND CELL FRAGMENTS
- MORE THAN 99 % RED BLOOD CELLS
- LESS THAN 1 % WHITE BLOOD CELLS AND PLATELETS
IF A SAMPLE OF BLOOD IS CENTRIFUGED OR SPUN IN A GLASS TUBE
- THE RED BLOOD CELLS SINK TO THE BOTTOM OF THE TUBE
- LIGHT WEIGHT PLASMA FORMS A LAYER ON TOP
- WBCs AND PLATELETS FORM A VERY THIN BUFFY COAT LAYER BETWEEN
- AS THEY ARE LESS DENSE THAN RBCs AND MORE DENSE THAN PLASMA
BLOOD PLASMA
STRAW COLORED LIQUID WHEN FORMED ELEMENTS ARE REMOVED FROM THE BLOOD
COMPONENTS
1. WATER- 91.5%
2. SOLUTES- 8.5 %
a. PROTEINS- 7%
b. OTHERS- 1.5 %
1. WATER
- LIQUID PORTION OF BLOOD
- ACTS AS SOLVENT AND SUSPENDING MEDIUM FOR COMPONENTS OF BLOOD
- ABSORBS
- TRANSPORTS
- RELEASES
HEAT
2a. PLASMA PROTEINS
- PROTEINS CONFINED TO BLOOD
A. ALBUMINS - 54%
- SMALLEST
- MOST NUMEROUS
- PRODUCED BY HEPATOCYTES
function
- transport proteins for several steroid hormones and
for fatty acids
B. GLOBULINS - 38 5
- PRODUCED BY HEPATOCYTES AND
PLASMA CELLS
a. ANTIBODIES- IMMUNOGLOBULINS
- HELP ATTACK VIRUSES AND BACTERIA
b. ALPHA AND BETA GLOBULINS
- TRANSPORT IRON
LIPIDS
FAT- SOLUBLE VITAMINS
C. FIBRINOGEN - 7 %
- PRODUCED BY HEPATOCYTES
- ESSENTIAL ROLE IN BLOOD CLOTTING
2b. OTHER SOLUTES
1. ELECTROLYTES
- INORGANIC SALTS
CATIONS- POSITIVELY CHARGED IONS- Na+, K+, Ca2+' Mg2+
ANIONS- NEGATIVELY CHARGED IONS- Cl-, HCO3-, HPO42-, SO4 2-
function
- HELP MAINTAIN OSMOTIC PRESSURE
- PLAY ESSENTIAL ROLES IN CELL FUNCTIONS
2. NUTRIENTS
- PRODUCTS OF DIGESTION
- PASS INTO BLOOD FOR DISTRIBUTION TO ALL BODY CELLS
INCLUDE
AMINO ACIDS FROM PROTEINS
GLUCOSE FROM CARBOHYDRATES
FATTY ACIDS AND GLYCEROL FROM TRIGLYCERIDES
VITAMINS
MINERALS
3. GASES
- OXYGEN
CARBON DIOXIDE
- NITROGEN
MORE OXYGEN - ASSOCIATED WITH HEMOGLOBIN INSIDE RBC
MORE CARBON DIOXIDE- IS DISSOLVED IN PLASMA
4. REGULATORY SUBSTANCES
a. ENZYMES
- PRODUCED BY BODY CELLS
- CATALYZE CHEMICAL REACTION
b. HORMONES
- PRODUCED BY ENDOCRINE GLAND
- REGULATE
METABOLISM
GROWTH
DEVELOPMENT
c. VITAMINS
- COFACTORS FOR ENZYMATIC REACTIONS
5. WASTE PRODUCTS
- MOST ARE BREAK DOWN PRODUCTS OF PROTEIN METABOLISM
- ARE CARRIED TO ORGANS OF EXCRETION
INCLUDE
UREA
URIC ACID
CREATINE
CREATININE
BILIRUBIN
AMMONIA
19. CARDIOVASCULAR SYSTEM - THE BLOOD INTRODUCTION
19. CARDIOVASCULAR SYSTEM - THE BLOOD
INTRODUCTION
BLOOD AND HOMEOSTASIS
1. BLOOD CONTRIBUTES TO HOMEOSTASIS BY
TRANSPORTING OXYGEN
CARBON DIOXIDE
NUTRIENTS
HORMONES
TO AND FROM OUR BODY CELLS
2. HELPS REGULATE BODY pH
AND TEMPERATURE
3. PROVIDE PROTECTION AGAINST DISEASE THROUGH
- PHAGOCYTOSIS
AND
- PRODUCTION OF ANTIBODIES
CARDIOVASCULAR SYSTEM
CARDIO = HEART
VASCULAR = BLOOD VESSELS
CONSISTS OF 3 INTERRELATED COMPONENETS
- HEART
- BLOOD
- BLOOD VESSELS
HEMATOLOGY
HEMA- / HEMATO- = BLOOD
- LOGY = STUDY OF
THE BRANCH OF SCIENCE CONCERNED WITH THE STUDY OF
- BLOOD
- BLOOD - FORMING TISSUES
- THE DISORDERS ASSOCIATED WITH THEM
INTRODUCTION
BLOOD AND HOMEOSTASIS
1. BLOOD CONTRIBUTES TO HOMEOSTASIS BY
TRANSPORTING OXYGEN
CARBON DIOXIDE
NUTRIENTS
HORMONES
TO AND FROM OUR BODY CELLS
2. HELPS REGULATE BODY pH
AND TEMPERATURE
3. PROVIDE PROTECTION AGAINST DISEASE THROUGH
- PHAGOCYTOSIS
AND
- PRODUCTION OF ANTIBODIES
CARDIOVASCULAR SYSTEM
CARDIO = HEART
VASCULAR = BLOOD VESSELS
CONSISTS OF 3 INTERRELATED COMPONENETS
- HEART
- BLOOD
- BLOOD VESSELS
HEMATOLOGY
HEMA- / HEMATO- = BLOOD
- LOGY = STUDY OF
THE BRANCH OF SCIENCE CONCERNED WITH THE STUDY OF
- BLOOD
- BLOOD - FORMING TISSUES
- THE DISORDERS ASSOCIATED WITH THEM
LIPIDS
LIPIDS
lip= fat
- Makes up 18 - 25 % of body mass in lean adults.
-Contain CARBON, HYDROGEN and OXYGEN.
But they do not have a 2:1 ratio of hydrogen to oxygen
LIPIDS ARE HYDROPHILIC
The proportion of electronegative oxygen atoms in lipids is usually smaller than carbohydrates.
- so there are fewer polar covalent bonds.
As a result , most lipids are insoluble in polar solvents such as water.
Only the smallest lipids (some fatty acids ) can dissolve in watery blood plasma.
LIPOPROTEINS
To become more soluble in blood plasma, lipidn molecules join with hydrophilic protein molecules.
The resulting lipid- protein complexes are called lipoproteins.
-Are soluble
- because proteins are outside and
lipids are on the inside.
LIPID FAMILY
1. FATTY ACIDS
2. TRIGLYCERIDES
3. PHOSPHOLIPIDS
4. STEROIDS
5. EICOSANOIDS
6. OTHERS
1. FATTY ACIDS
- Used to synthesize triglycerides and phospholipids.
- catabolized to generate ATP.
- consists of
- a carboxyl group
and
- a hydrocarbon group chain.
- can be either saturated or unsaturated.
1. SATURATED FATTY ACID
- Contains only single covalent bond between carbon atoms of the hydrocarbon chain.
Because they lack double bonds, each carbon atom of hydrocarbon chain is saturated with hydrogen atom.
eg. Palmitic acid C15H31COOH.
2. UNSATURATED FATTY ACID
Contains
1 orv more double covalent bonds between carbon atoms of the hydrocarbon chain.
Thus the fatty acid is not completely saturated with hydrogen atoms.
eg. Oleic acid C17H33COOH
The unsaturated fatty acid has a KINK/ BEND at the site of the double bond.
MONOUNSATURATED FATTY ACID
If fatty acid has just 1 double bond in hydrocarbon chain.
eg. Oleic acid.
POLYUNSATURATED FATTY ACID - PUFA
If fatty acid more than 1 double bond in hydrocarbon chain.
- contains more than a kink / bend
eg. Omega-3 fatty acids
FATTY ACIDS IN HEALTH AND DISEASE
ESSENTIAL FATTY ACIDS
- A group of fatty acids essential to human health
- Cannot be made by human body.
- Must be obtained from foods or supplements.
MOST IMPORTANT ESSENTIAL FATTY ACIDS
1. OMEGA-3 FATTY ACIDS
2. OMEGA-6 FATTY ACIDS
3. cis- FATTY ACIDS
OMEGA-3 and OMEGA-6 FATTY ACIDS
-Poly unsaturated fatty acids
-Believed to work together to promote health.
1. may have a protective effect against heart disease and stroke.
- by lowering total cholesterol
- raising HDL / good cholesterol.
- lowering LDL / bad cholesterol.
2. Decrease bone loss
- by increasing calcium utilization by body.
3. Reduce symptoms of arthritis due to inflammation
4. Improve certain skin disorders
- psoriasis
- eczema
- acne
5. Improve mental functions.
PRIMARY SOURCES OF OMEGA-3 FATTY ACIDS
- Flax seed
- Fatty fish
- Fish oils
- Walnuts
- Oils that have large amounts of poly unsaturated fatty acids.
PRIMARY SOURCES OF OMEGA-6 FATTY ACIDS
- Processed foods
- cereals
- breads
- white rice
- Baked goods
- Eggs
- Oils that have large amounts of poly unsaturated fatty acids.
- Meat especially organ meats such as liver.
CIS- FATTY ACIDS
- An unsaturated fatty acid with with hydrogen atom on either side of the double bond are on the same side of the unsaturated fatty acid
- are nutritionally beneficial.
- used by the body to produce hormone like regulators and cell membrane
When cis- fatty acids are heated , pressurized and combined with a catalyst (usually nickel) by hydrogenation
- they are changed to unhealthy trans- fatty acids
TRANS- FATTY ACIDS
Hydrogen atoms are on opposite sides of the double bond of an unsaturated fatty acid
Hydrogenation is used by manufacturers to make vegetable oils solid at room temperature
-and less likely to turn rancid.
Hydrogenated / trans- fatty acids are common in
- commercially baked goods
- crackers
- cakes
- cookies
- salty snack foods
- some margarine's
fried foods- donuts, french fries
When oil is used fro frying, and if the oils reused,
- cis- fatty acids are converted to trans- fatty acids.
If a product label contains the word hydrogenated or partially hydrogenated,
- then the product contains trans - fatty acids
ADVERSE EFFECTS OF TRANS- FATTY ACIDS
1. Increase in total cholesterol.
2. Decrease in HDL.
3. Increase in LDL.
4. Increase in triglycerides.
5. Increase risk of heart diseases and other cardio-vascular diseases.
2. TRIGLYCERIDES / TRIGLYCEROLS
tri=3
The most plentiful lipids in our body and diet.
- consists of 2 types of building blocks.
- a single glycerol molecule
- 3 fatty acid molecules.
- a 3- carbon glycerol molecule forms the backbone of a triglyceride.
- 3 fatty acids are attached by Dehydration synthesis reactions
- one to each carbon of the glycerol backbone.
- The chemical bond formed where each water molecule is removed is an ester linkage
The reverse reaction- HYDROLYSIS-
- breaks down a single molecule of a triglyceride into 3 fatty acids
HYDROLYSIS
TRIGLYCERIDES - 3 FATTY ACIDS
FUNCTIONS
- Body's most highly concentrated form of chemical energy .
- provide more than twice as much energy per gram as do carbohydrates and proteins.
- Our capacity to store triglycerides in adipose tissue is unlimited for practical purposes.
- Excess carbohydrates, proteins, fats and oils are deposited in adipose tissues as triglycerides.
FAT
Triglycerides that us solid at room temperature.
- Fatty acids of a fat are mostly saturated.
- these lack double bonds in their hydrocarbon chains,
- they can closely pack together and solidify at room temperature.
SATURATED FAT
- A fat that mainly consists of saturated fatty acids.
- Occur
- mostly in meats especially red meats.
- nonskim dairy products
- whole milk
- cheese
- butter
- in cocoa butter
- palm oil
- coconut oil
Diets that conatin large amounts of saturate dfats are associated with disorders sucha s heart disease and colorectal cancer.
OIL
Triglycerides that is liquid at room temperature .
-Fats of oil are mostly unsaturated.
The kinks at the sites of the double bonds prevent unsaturated fatty acids of an oil from closely packing together and solidifying.
The fatty acids of an oil can be either
- monounsaturated
OR
- polyunsaturated
MONOUNSATURATED FATS
- Contain triglycerides with monounsaturated fatty acid.
eg.
olive oil
peanut oil
canola il
most nuts
avocados
POLYUNSATURATED FATS
- Contain triglyceride that mostly consist of polyunsaturated fatty acids.
eg.
corn oil
safflower oil
sunflower oil
soyabean oil
fatty fish - salmon
- tuna
- mackerel
Both monounsaturated and polyunsaturated fats are believed to decrease the risk of heart disease.
3. PHOSPHOLIPIDS
- Lipids that contain phosphorus.
- Major lipid component of cell membrane.
- Have a glycerol backbone
and
2 fatty acid chains attached to the first carbon atom
HEAD POSITION
-In the third position a phosphate group (PO4 3-) links a small charged group that usually contains nitrogen to the backbone.
-Is polar.
- Can form hydrogen bonds with water molecules.
TAIL POSITION
- the 2 fatty acids
- Non polar
- Can interact only with other lipids.
AMPHIPATHIC
amphi= on both sides
-pathic= feeling
- Molecules that have both polar and non polar parts.
- Amphipathic phospholipids line up tail to tail in a double row to make up much of the membrane that surrounds each cell.
4. STEROIDS
- Lipids that have 4 rings of carbon atoms.
- Body cells synthesize other steroids from cholesterol
- which has a large non polar region.
- consists of 4 rings and a hydrocarbon tail.
'
COMMON STEROIDS IN BODY
These are known as STEROLS
- because they also have at least 1 hydroxyl(alcohol) group -OH.
The polar hydroxyl groups make sterol weakly amphipathic.
a. CHOLESTEROL
-Minor component of all animal cell membranes.
- Precursor of bile salts
Vitamin D
steroid hormone
b. BILE SALTS
- Needed for lipid digestion
and absorption of dietary lipids.
c. Vitamin D
- Helps to regulate calcium level in body.
- Needed for bone growth and repair.
d. Adrenocortical hormones
-Help regulate metabolism
resistance to stress
salt and water balance.
eg.
Cortisol- for maintaining normal blood sugar levels.
e. SEX HORMONES
- Stimulate reproductive functions and sexual characteristics
eg. estrogen
testosterone
5. EICOSANOIDS
eicosan= 20
- Lipids derived from a 20 carbon fatty acid called arachidonic acid.
2 PRINCIPAL CLASSES
A. PROSTAGLANDIN
B. LEUKOTRIENES
A. Prostaglandin
- a. modify responses to hormones.
- b. contributes to inflammatory response.
- c. influence formation of blood clots.
- d. regulate body temperature.
- e. dilate air ways to the lungs.
- f. prevent stomach ulcers.
B. Leukotrienes
- participate in allergic and inflammatory responses.
6. OTHERS
A. FAT SOLUBLE VITAMINS
B. LIPOPROTEINS
a. FAT SOLUBLE VITAMINS
1. CAROTENES
- Yellow - orange pigments in egg yolk
carrots
tomatoes
- needed for synthesis of Vitamin- A
- functions as anti oxidants.
2. VITAMINS A,D,E,K
a. VITAMIN-A
- Used to make visual pigments in the eye.
b. Vitamin D
- Helps to regulate calcium level in body.
- Needed for bone growth and repair.
c. VITAMIN- E
- Promotes wound healing.
- prevents tissue scarring.
- contributes to the normal structure and function of nervous system.
- functions as an antioxidant.
d. VITAMIN-K
- Required for synthesis of blood clotting proteins.
B. LIPOPROTEINS
-Transports lipids in the blood
- Carry triglycerides and cholesterol to tissues.
- Remove excess cholesterol from the blood.
lip= fat
- Makes up 18 - 25 % of body mass in lean adults.
-Contain CARBON, HYDROGEN and OXYGEN.
But they do not have a 2:1 ratio of hydrogen to oxygen
LIPIDS ARE HYDROPHILIC
The proportion of electronegative oxygen atoms in lipids is usually smaller than carbohydrates.
- so there are fewer polar covalent bonds.
As a result , most lipids are insoluble in polar solvents such as water.
Only the smallest lipids (some fatty acids ) can dissolve in watery blood plasma.
LIPOPROTEINS
To become more soluble in blood plasma, lipidn molecules join with hydrophilic protein molecules.
The resulting lipid- protein complexes are called lipoproteins.
-Are soluble
- because proteins are outside and
lipids are on the inside.
LIPID FAMILY
1. FATTY ACIDS
2. TRIGLYCERIDES
3. PHOSPHOLIPIDS
4. STEROIDS
5. EICOSANOIDS
6. OTHERS
1. FATTY ACIDS
- Used to synthesize triglycerides and phospholipids.
- catabolized to generate ATP.
- consists of
- a carboxyl group
and
- a hydrocarbon group chain.
- can be either saturated or unsaturated.
1. SATURATED FATTY ACID
- Contains only single covalent bond between carbon atoms of the hydrocarbon chain.
Because they lack double bonds, each carbon atom of hydrocarbon chain is saturated with hydrogen atom.
eg. Palmitic acid C15H31COOH.
2. UNSATURATED FATTY ACID
Contains
1 orv more double covalent bonds between carbon atoms of the hydrocarbon chain.
Thus the fatty acid is not completely saturated with hydrogen atoms.
eg. Oleic acid C17H33COOH
The unsaturated fatty acid has a KINK/ BEND at the site of the double bond.
MONOUNSATURATED FATTY ACID
If fatty acid has just 1 double bond in hydrocarbon chain.
eg. Oleic acid.
POLYUNSATURATED FATTY ACID - PUFA
If fatty acid more than 1 double bond in hydrocarbon chain.
- contains more than a kink / bend
eg. Omega-3 fatty acids
FATTY ACIDS IN HEALTH AND DISEASE
ESSENTIAL FATTY ACIDS
- A group of fatty acids essential to human health
- Cannot be made by human body.
- Must be obtained from foods or supplements.
MOST IMPORTANT ESSENTIAL FATTY ACIDS
1. OMEGA-3 FATTY ACIDS
2. OMEGA-6 FATTY ACIDS
3. cis- FATTY ACIDS
OMEGA-3 and OMEGA-6 FATTY ACIDS
-Poly unsaturated fatty acids
-Believed to work together to promote health.
1. may have a protective effect against heart disease and stroke.
- by lowering total cholesterol
- raising HDL / good cholesterol.
- lowering LDL / bad cholesterol.
2. Decrease bone loss
- by increasing calcium utilization by body.
3. Reduce symptoms of arthritis due to inflammation
4. Improve certain skin disorders
- psoriasis
- eczema
- acne
5. Improve mental functions.
PRIMARY SOURCES OF OMEGA-3 FATTY ACIDS
- Flax seed
- Fatty fish
- Fish oils
- Walnuts
- Oils that have large amounts of poly unsaturated fatty acids.
PRIMARY SOURCES OF OMEGA-6 FATTY ACIDS
- Processed foods
- cereals
- breads
- white rice
- Baked goods
- Eggs
- Oils that have large amounts of poly unsaturated fatty acids.
- Meat especially organ meats such as liver.
CIS- FATTY ACIDS
- An unsaturated fatty acid with with hydrogen atom on either side of the double bond are on the same side of the unsaturated fatty acid
- are nutritionally beneficial.
- used by the body to produce hormone like regulators and cell membrane
When cis- fatty acids are heated , pressurized and combined with a catalyst (usually nickel) by hydrogenation
- they are changed to unhealthy trans- fatty acids
TRANS- FATTY ACIDS
Hydrogen atoms are on opposite sides of the double bond of an unsaturated fatty acid
Hydrogenation is used by manufacturers to make vegetable oils solid at room temperature
-and less likely to turn rancid.
Hydrogenated / trans- fatty acids are common in
- commercially baked goods
- crackers
- cakes
- cookies
- salty snack foods
- some margarine's
fried foods- donuts, french fries
When oil is used fro frying, and if the oils reused,
- cis- fatty acids are converted to trans- fatty acids.
If a product label contains the word hydrogenated or partially hydrogenated,
- then the product contains trans - fatty acids
ADVERSE EFFECTS OF TRANS- FATTY ACIDS
1. Increase in total cholesterol.
2. Decrease in HDL.
3. Increase in LDL.
4. Increase in triglycerides.
5. Increase risk of heart diseases and other cardio-vascular diseases.
2. TRIGLYCERIDES / TRIGLYCEROLS
tri=3
The most plentiful lipids in our body and diet.
- consists of 2 types of building blocks.
- a single glycerol molecule
- 3 fatty acid molecules.
- a 3- carbon glycerol molecule forms the backbone of a triglyceride.
- 3 fatty acids are attached by Dehydration synthesis reactions
- one to each carbon of the glycerol backbone.
- The chemical bond formed where each water molecule is removed is an ester linkage
The reverse reaction- HYDROLYSIS-
- breaks down a single molecule of a triglyceride into 3 fatty acids
HYDROLYSIS
TRIGLYCERIDES - 3 FATTY ACIDS
FUNCTIONS
- Body's most highly concentrated form of chemical energy .
- provide more than twice as much energy per gram as do carbohydrates and proteins.
- Our capacity to store triglycerides in adipose tissue is unlimited for practical purposes.
- Excess carbohydrates, proteins, fats and oils are deposited in adipose tissues as triglycerides.
FAT
Triglycerides that us solid at room temperature.
- Fatty acids of a fat are mostly saturated.
- these lack double bonds in their hydrocarbon chains,
- they can closely pack together and solidify at room temperature.
SATURATED FAT
- A fat that mainly consists of saturated fatty acids.
- Occur
- mostly in meats especially red meats.
- nonskim dairy products
- whole milk
- cheese
- butter
- in cocoa butter
- palm oil
- coconut oil
Diets that conatin large amounts of saturate dfats are associated with disorders sucha s heart disease and colorectal cancer.
OIL
Triglycerides that is liquid at room temperature .
-Fats of oil are mostly unsaturated.
The kinks at the sites of the double bonds prevent unsaturated fatty acids of an oil from closely packing together and solidifying.
The fatty acids of an oil can be either
- monounsaturated
OR
- polyunsaturated
MONOUNSATURATED FATS
- Contain triglycerides with monounsaturated fatty acid.
eg.
olive oil
peanut oil
canola il
most nuts
avocados
POLYUNSATURATED FATS
- Contain triglyceride that mostly consist of polyunsaturated fatty acids.
eg.
corn oil
safflower oil
sunflower oil
soyabean oil
fatty fish - salmon
- tuna
- mackerel
Both monounsaturated and polyunsaturated fats are believed to decrease the risk of heart disease.
3. PHOSPHOLIPIDS
- Lipids that contain phosphorus.
- Major lipid component of cell membrane.
- Have a glycerol backbone
and
2 fatty acid chains attached to the first carbon atom
HEAD POSITION
-In the third position a phosphate group (PO4 3-) links a small charged group that usually contains nitrogen to the backbone.
-Is polar.
- Can form hydrogen bonds with water molecules.
TAIL POSITION
- the 2 fatty acids
- Non polar
- Can interact only with other lipids.
AMPHIPATHIC
amphi= on both sides
-pathic= feeling
- Molecules that have both polar and non polar parts.
- Amphipathic phospholipids line up tail to tail in a double row to make up much of the membrane that surrounds each cell.
4. STEROIDS
- Lipids that have 4 rings of carbon atoms.
- Body cells synthesize other steroids from cholesterol
- which has a large non polar region.
- consists of 4 rings and a hydrocarbon tail.
'
COMMON STEROIDS IN BODY
These are known as STEROLS
- because they also have at least 1 hydroxyl(alcohol) group -OH.
The polar hydroxyl groups make sterol weakly amphipathic.
a. CHOLESTEROL
-Minor component of all animal cell membranes.
- Precursor of bile salts
Vitamin D
steroid hormone
b. BILE SALTS
- Needed for lipid digestion
and absorption of dietary lipids.
c. Vitamin D
- Helps to regulate calcium level in body.
- Needed for bone growth and repair.
d. Adrenocortical hormones
-Help regulate metabolism
resistance to stress
salt and water balance.
eg.
Cortisol- for maintaining normal blood sugar levels.
e. SEX HORMONES
- Stimulate reproductive functions and sexual characteristics
eg. estrogen
testosterone
5. EICOSANOIDS
eicosan= 20
- Lipids derived from a 20 carbon fatty acid called arachidonic acid.
2 PRINCIPAL CLASSES
A. PROSTAGLANDIN
B. LEUKOTRIENES
A. Prostaglandin
- a. modify responses to hormones.
- b. contributes to inflammatory response.
- c. influence formation of blood clots.
- d. regulate body temperature.
- e. dilate air ways to the lungs.
- f. prevent stomach ulcers.
B. Leukotrienes
- participate in allergic and inflammatory responses.
6. OTHERS
A. FAT SOLUBLE VITAMINS
B. LIPOPROTEINS
a. FAT SOLUBLE VITAMINS
1. CAROTENES
- Yellow - orange pigments in egg yolk
carrots
tomatoes
- needed for synthesis of Vitamin- A
- functions as anti oxidants.
2. VITAMINS A,D,E,K
a. VITAMIN-A
- Used to make visual pigments in the eye.
b. Vitamin D
- Helps to regulate calcium level in body.
- Needed for bone growth and repair.
c. VITAMIN- E
- Promotes wound healing.
- prevents tissue scarring.
- contributes to the normal structure and function of nervous system.
- functions as an antioxidant.
d. VITAMIN-K
- Required for synthesis of blood clotting proteins.
B. LIPOPROTEINS
-Transports lipids in the blood
- Carry triglycerides and cholesterol to tissues.
- Remove excess cholesterol from the blood.
ORGANIC COMPOUNDS and FUNCTIONAL GROUP
ORGANIC COMPOUNDS and FUNCTIONAL GROUP
- Always contain Carbon
- Usually contain Hydrogen
-Always have covalent bonds.
- Many are made up of long chain of Carbon atoms
-Make up 38- 43 % of the human body.
- Have unique characteristics that allow them to carry out complex functions.
IMPORTANT CATEGORIES
1. CARBOHYDRATES
2. LIPIDS
3. PROTEINS
4. NUCLEIC ACIDS
5. ATP
CARBON AND FUNCTIONAL GROUPS
Carbon can form bond with 1 to 1000 carbon atoms to produce large molecules that can have different shapes
CARBON SKELETON
The chain of carbon atoms in an organic molecule.
HYDROCARBONS
Carbons bonded to Hydrogen atoms.
FUNCTIONAL GROUP
An atom or group of atoms
- that replaces hydrogen in an organic compound
- that defines the structure of a family of compounds
- determines the properties of the family.
Each type of functional group has a specific arrangement of atoms that confers characteristic chemical properties on the organic molecules attached to it.
MAJOR FUNCTIONAL GROUPS
R = VARIABLE GROUP.
1. Hydroxyl R - O - H
2. Sulfhydryl R - S -H
3. Carbonyl R - C=O.
4. Carboxyl R - COOH
5. Ester R - COO
6. Phosphate R - PO4 2–
7. Amino R - NH2
1. Hydroxyl -OH
R - O -H
- Alcohols
- Contain an -OH group
-Is polar
and
hydrophilic due to its electronegative Oxygen atom.
- Molecules with many -OH groups
- dissolve easily in water
eg. Ethanol - C2H5OH
2. Sulfhydryl -SH
R - S - H
THIOLS or MERCAPTANS
-Have an -SH group
-Is polar and hydrophilic
- due to its electronegative Sulphur atom.
eg.Methyl mercaptan CH3-SH
Certain amino acids contain -SH groups
-which stabilize the shape of proteins
eg . Cysteine
3. Carbonyl R - C=O.
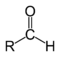 ALDEHYDES R- CHO
ALDEHYDES R- CHO
A. KETONES R - CO - R
-Contains a carbonyl group with in the carbon skeleton.
The carbonyl group is polar and hydrophilic
- due to its electronegative Oxygen atom.
eg. Acetone.
B. ALDEHYDE -CHO
-Have a carbonyl group at the end of the carbon skeleton.
eg. acetaldehyde
4. Carboxyl - COOH

CARBOXYLIC ACID -COOH
Carboxylic acids contain a carboxyl group at the end of the carbon skeleton.
All amino acids have a -COOH group at 1 end.
The negatively charged form predominates at the pH of body cells
- is hydrophilic.
eg. Acetic acid CH3COOH
5. ESTER -COO

-Predominate in dietary fats and oils
- Occur in our body in trigycerides
eg. Aspirin
-is an ester of Salicylic acid
- a pain relieving molecule
- found in the bark of the willow tree.
6. PHOSPHATE -PO4 2-

- Contain a phosphate group
- Is very hydrophilic due to dual negative charges
eg. ATP
7. Amino -NH2


-Amines have an -NH2 group.
- Can act as a base.
- Pick up a Hydrogen ion
- giving the amino group a positive charge .
At the pH of body fluids,
most amino groups have a charge of 1+.
All amino acids have an amino group at 1 end.
MACROMOLECULES
macro= large
- Small organic molecules can combine into very large molecules.
- Are usually polymers
eg. Carbohydrates
A. MONOMERS
B. POLYMERS
MONOMERS
mono= 1
- mers = parts
- small building block molecules.
POLYMERS
poly= many
A polymer is a large molecule formed by the covalent bonding of many identical or similar monomers.
The reaction that joins 2 monomers is normally a dehydration synthesis.
DEHYDRATION SYNTHESIS REACTION
In this type of reaction,
- a hydrogen atom is removed from 1 monomer
- a hydroxylgroup is removed from other
- to form a molecule of water.
ISOMERS
iso= equal
Molecules that have same molecular formula
but different structure
eg.
Molecular formula for glucose and fructose are both C6H12O6
But structural formula differs giving different chemical properties
- because relative positions of oxygen and carbon atoms are different.

-
- Always contain Carbon
- Usually contain Hydrogen
-Always have covalent bonds.
- Many are made up of long chain of Carbon atoms
-Make up 38- 43 % of the human body.
- Have unique characteristics that allow them to carry out complex functions.
IMPORTANT CATEGORIES
1. CARBOHYDRATES
2. LIPIDS
3. PROTEINS
4. NUCLEIC ACIDS
5. ATP
CARBON AND FUNCTIONAL GROUPS
Carbon can form bond with 1 to 1000 carbon atoms to produce large molecules that can have different shapes
CARBON SKELETON
The chain of carbon atoms in an organic molecule.
HYDROCARBONS
Carbons bonded to Hydrogen atoms.
FUNCTIONAL GROUP
An atom or group of atoms
- that replaces hydrogen in an organic compound
- that defines the structure of a family of compounds
- determines the properties of the family.
Each type of functional group has a specific arrangement of atoms that confers characteristic chemical properties on the organic molecules attached to it.
MAJOR FUNCTIONAL GROUPS
R = VARIABLE GROUP.
1. Hydroxyl R - O - H
2. Sulfhydryl R - S -H
3. Carbonyl R - C=O.
4. Carboxyl R - COOH
5. Ester R - COO
6. Phosphate R - PO4 2–
7. Amino R - NH2
1. Hydroxyl -OH
R - O -H
- Alcohols
- Contain an -OH group
-Is polar
and
hydrophilic due to its electronegative Oxygen atom.
- Molecules with many -OH groups
- dissolve easily in water
eg. Ethanol - C2H5OH
2. Sulfhydryl -SH
R - S - H
THIOLS or MERCAPTANS
-Have an -SH group
-Is polar and hydrophilic
- due to its electronegative Sulphur atom.
eg.Methyl mercaptan CH3-SH
Certain amino acids contain -SH groups
-which stabilize the shape of proteins
eg . Cysteine
3. Carbonyl R - C=O.
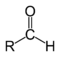
A. KETONES R - CO - R
-Contains a carbonyl group with in the carbon skeleton.
The carbonyl group is polar and hydrophilic
- due to its electronegative Oxygen atom.
eg. Acetone.
B. ALDEHYDE -CHO
-Have a carbonyl group at the end of the carbon skeleton.
eg. acetaldehyde
4. Carboxyl - COOH
CARBOXYLIC ACID -COOH
Carboxylic acids contain a carboxyl group at the end of the carbon skeleton.
All amino acids have a -COOH group at 1 end.
The negatively charged form predominates at the pH of body cells
- is hydrophilic.
eg. Acetic acid CH3COOH
5. ESTER -COO
-Predominate in dietary fats and oils
- Occur in our body in trigycerides
eg. Aspirin
-is an ester of Salicylic acid
- a pain relieving molecule
- found in the bark of the willow tree.
6. PHOSPHATE -PO4 2-

- Contain a phosphate group
- Is very hydrophilic due to dual negative charges
eg. ATP
7. Amino -NH2


-Amines have an -NH2 group.
- Can act as a base.
- Pick up a Hydrogen ion
- giving the amino group a positive charge .
At the pH of body fluids,
most amino groups have a charge of 1+.
All amino acids have an amino group at 1 end.
MACROMOLECULES
macro= large
- Small organic molecules can combine into very large molecules.
- Are usually polymers
eg. Carbohydrates
A. MONOMERS
B. POLYMERS
MONOMERS
mono= 1
- mers = parts
- small building block molecules.
POLYMERS
poly= many
A polymer is a large molecule formed by the covalent bonding of many identical or similar monomers.
The reaction that joins 2 monomers is normally a dehydration synthesis.
DEHYDRATION SYNTHESIS REACTION
In this type of reaction,
- a hydrogen atom is removed from 1 monomer
- a hydroxylgroup is removed from other
- to form a molecule of water.
ISOMERS
iso= equal
Molecules that have same molecular formula
but different structure
eg.
Molecular formula for glucose and fructose are both C6H12O6
But structural formula differs giving different chemical properties
- because relative positions of oxygen and carbon atoms are different.

-
Langganan:
Komentar (Atom)

 KETONES R - CO - R
KETONES R - CO - R